We all know the primary components of our favorite sudsy brewskies…grain, hops, yeast, and water; however, the DNA of our beer is often a little more complex than we first think.
Throughout the brewing process, a number of other, lesser-known substances come into play, one of which is the subject of today’s focus, maltose.
BEER DROP: Boxes of beer from Award-winning microbreweries → Join The Club
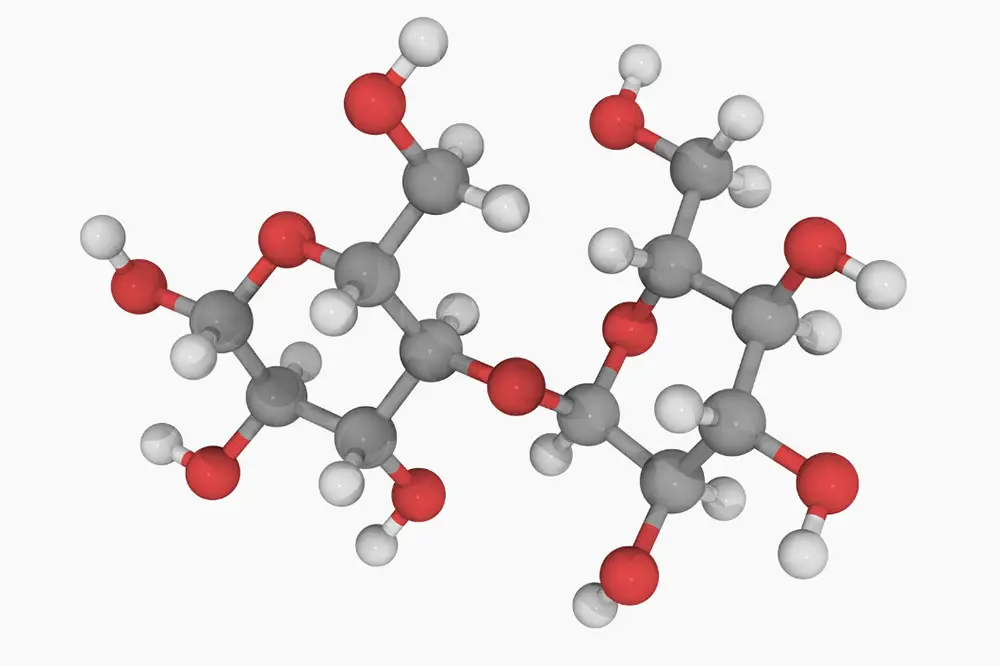
I know what you’re thinking…what the heck is maltose? Well, simply put, it’s a form of sugar created during the malting process. In more technical terms (if you’ll please don your lab coat and safety goggles), it’s a disaccharide composed of two units of glucose joined at the hip by a 1,4 bond.
Has this sweet little taster piqued your interest? Fantastic, let’s continue with some more in-depth analysis of this nebulous byproduct.
What Precisely Is Maltose?
As I’ve already touched upon, Maltose is a sugar of sorts (hence the “ose” suffix), but it’s nowhere near as sweet as the granulated sugar we put far too much of on our Weetabix in the morning.
It’s actually rather muted, and despite the name sounding like I just made it up on the spot, it’s actually been around for as long as grain has grown and rain has fallen.
It’s been around since before we primates were walking on two feet, and long before we had the novel idea to make beer and consequently knock ourselves back down, counteracting millions of years of evolution in the process — sorry, Darwin.
Maltose is a byproduct of the malting process, which is the act of wetting grain and allowing it to sprout. This is done to break down the grain into enzymes and sugars, making a substance that’s far more receptive to the fermentation process, which, as you know, is what puts the funny in our funny juice.
When maltose reaches our intestine, its constituent glucose units split apart, ready to be broken down for use as an energy source or to be held as glycogen for later use.
Or in more technical terms – if you’ll please put your lab coats and safety goggles back on – the disaccharide composition splits into monosaccharides once the glycosidic linkage is broken.
Disaccharides – What Are They?
In a macroscopic sense, disaccharides are one of the four chemical categorizations of carbohydrates, the others being monosaccharides, oligosaccharides, and polysaccharides.
As I’m sure you’ve already deduced, disaccharides are formed when two monosaccharides fuse together. The bond holding them together is called friendship — just kidding; it’s actually called a glycosidic linkage (not nearly as heart-warming, but that’s science for you).
What Common Food Items Contain Maltose?
It’s actually kind of shocking that not many people know about maltose because this stuff is EVERYWHERE! Seriously…check out this list of some of its most common hiding places.
- Bread
- Crackers
- Breakfast Cereal
- Wheat
- Barley
- Cornmeal
- Honey
- Candy
- Energy Bars
- Molasses
- Peaches
- Pairs
- Yams
Did that just blow your mind or what? When you started learning about Maltose a few minutes ago, I bet you weren’t expecting to hear that you’ve probably got oodles of the stuff inside you right now.
The main offenders in terms of maltose content are grains — we’re talking rye, corn, barley, and wheat. But as you’ve seen on my list, it’s also present in a lot of other food items, such as viscous sweetener, molasses
It can be found in any foods created by breaking down starch, such as malt shakes and, of course, Maltesers. What’s more, it occurs naturally in any food or drink that’s made via the fermentation of starch.
Before we move on, one interesting thing to note is that not all foods start out packed full of maltose. Take the humble yam, for instance. Raw, they contain absolutely zero maltose. It’s only once they’re cooked that that maltose creeps into the equation.
What Do We Use Maltose For?
We humans treat maltose kind of like a knock-off sugar. It’s sort of a…why spend $5,000 on a genuine Gucci clutch, when you can buy one that looks identical from an imitation brand for $20…situation.
We use it as a cost-effective alternative to the white gold we’re stirring into our coffees every morning before work. It’s used to make candies and syrups, it’s used for cooking, and most importantly of all, it’s used to brew all our favorite alcoholic beverages, which brings me to my next point.
Maltose and Alcohol Production
Maltose makes up most of the digestible sugar used to activate the yeast and trigger the fermentation process, making it a key player in the brewing process of whiskey and beer.
Once the yeast has metabolized the maltose, we’re left with ethanol and carbon dioxide, the former of which, my friends, is good old-fashioned booze!
So you see, though maltose has been the subject of recent vilification by carb-counters, without it, we’d be watching the game sober, realizing that football ain’t half as exciting as we thought.
It’s formed alongside proteins as starch is broken down during the mashing process, which entails mixing crushed grains with warm water, creating a sweet, soft concoction, perfect for hungry yeast. The wort is then drawn from the mash and ferried over to fermentation chambers for, you’ve guessed it…fermentation.
The chief starch in regard to maltose production is known as beta amylase. It’s responsible for a whopping 40% of the wort’s carb content. To trigger optimal beta amylase activity during the mashing stage, the temperature of the concoction has to be kept between 60-65° C, thereby encouraging maltose production.
Maltose can also be added to the wort after the fact if necessary, even if it doesn’t share a native grain with the mash. You can mix rye with wheat or corn with barley…it doesn’t really matter.
Once the secondary maltose is distilled into a syrup using microbial enzymes, it can be introduced to the wort, significantly increasing the ethanol yield, a handy trick for brewers with limited mashing facilities.
What Is Metabolism
I’m going to be throwing this world around right, left, and center, so just to make sure you understand what I’m waffling on about, I thought I’d set aside this little space to explain what metabolism actually is.
Metabolism, in very general terms, is the collection of cellular chemical reactions in a living organism that extract and use the good stuff from another source in order to keep living.
For example, humans need to eat lots of food to survive, but it’s our metabolism that transfers the food we eat into energy. Without metabolism, food wouldn’t provide sustenance, and we would die.
Is Maltose A Reducing Sugar, And What Exactly Is A Reducing Sugar?
One of the major differentiators between regular old sucrose and the glucose-based alternative, maltose, is that maltose is a reducing sugar.
Reducing sugar is classified as any sugar with an open-chain composition corresponding to either an aldehyde or hemiacetal group.
Reducing Sugars Explained
I hope you’ve got your thinking cap on because this is going to get pretty cerebral. Reducing sugar is essentially just a carbohydrate with an anomeric carbon that contains a terminal aldehyde or ketone group (more on those in just a sec).
When mixed into a solution, they will react with surrounding molecules and oxidize.
Another way of putting it is that reducing sugar can give electrons to surrounding molecules, thereby changing the color and flavor of the solution as a whole.
You know that delicious golden-brown hue of bread? Well, that’s partly the work of reducing sugar reacting with proteins as it bakes — pretty neat, huh?
Regular old sucrose molecules have anomeric carbons, but they do not contain an OH group, and thus, cannot oxidize with other molecules.
Aldehyde Groups
I know I’m throwing some heady stuff your way right now, so let’s break it down. Thankfully, at this juncture, you can forget about ketone groups and focus up on aldehyde groups, as ketone groups are only present in fructose sugar.
An aldehyde group has a -CHO structure, and in this context, is essentially a dynamic group of natural compounds that make up a part of the maltose molecule.
However, this group isn’t there to start with, rather it’s formed when the free hemiacetal group of one of the glucose molecules opens up, allowing the donation of electrons to surrounding molecules. This hemiacetal opening can be triggered when the maltose molecules are mixed into a solution and gently heated.
Get it? No? I don’t blame you, I’m still trying to wrap my head around it. Basically…a reducing sugar has the ability to form chemical reactions with other molecules, but a non-reducing sugar will not be able to engage in such interactions.
Is Maltose a Pentose Sugar – Yes or No?
Pentose is a fancy way of saying a sugar is simple, meaning it is a monosaccharide composed of only five carbon atoms.
As maltose molecules are composed of two glucose units, each containing six carbon molecules, it cannot be considered a pentose sugar. In fact, its disaccharide structure prohibits such a classification before you even consider the atomic makeup of glucose.
Maltose is actually known to take the pyranose form, a blanket term describing disaccharides with a ring of six connected atoms (5 carbon and 1 oxygen) as well as any that also feature carbon atoms outside the core ring.
Does Maltose Have An Effect On Blood Sugar?
There has been very little research into the effects of maltose on the human body, but we can make assertions based on how it behaves when consumed.
As I mentioned earlier, once maltose hits the intestine, the two glucose units split and continue life as individuals. In light of this, it stands to reason that maltose would have the same effect on the body as glucose.
Glucose does cause an initial spike in blood sugar, but as the glucose is absorbed by the liver and passed on to the muscles, fat tissue, and other cellular locations, your blood sugar stabilizes to where it was before the glucose was consumed.
It’s only when we consume an excessive amount of glucose that it can have a negative impact on our blood sugar levels and, of course, our general health.
Maltose vs Table Sugar – Which Is Healthiest?
Considering the ubiquity of standard table sugar, you’d be forgiven for thinking it was the healthier option, but alas, no, it is not…at least we don’t think it is anyway.
Like maltose, table sugar is made up of dual-unit molecules, but unlike maltose, the units lack uniformity. Whereas maltose is composed of two glucose units, table sugar is made of one glucose unit and one fructose unit.

Fructose is much harder on the liver than glucose and, among other things, can increase the risk of liver disease, obesity, and high blood sugar.
So, hypothetically, maltose is the healthier sweetener, but without further testing, nobody can say for sure. The bottom line is that whichever sugar you’re using, it should only be consumed in moderation.
Is Maltose Bad For Your Health?
As maltose is composed entirely of glucose, it’s likely not actually that bad for your health. In fact, our bodies need glucose for energy; it’s our brain’s absolute favorite food! It allows our nerve cells and chemical messengers to communicate efficiently.
Without glucose, our blood sugar levels would plummet, leading to excess sweating, lethargy, dizziness, hunger, heart palpitations, the shakes, tingling lips, and general moodiness.
That said, too much glucose can damage the vessels that carry blood to the vital organs, increase the risk of heart and kidney disease, increase your chances of suffering a stroke, and can even affect your vision.
There might also be some unforeseen health risks involved with maltose consumption. We won’t know until more studies are carried out, but it seems extremely unlikely.
I know I’m flogging a dead horse at this point, but the key takeaway here is once again…everything in moderation.
Can Maltose Be Considered A Protein?
Protein is one of the fundamental nutrients we need to survive, along with carbohydrates, and fats. Maltose is a carbohydrate, so it cannot be considered a protein.
The function of the two nutrients does overlap at times, so it’s easy to confuse them, but they are distinct entities.
Carbohydrates are an energy source…that’s it. They give us that get up and go, put that skip in our step, and keep our brains in tip-top shape. Proteins, on the other hand, do provide energy, but only after their other chores are complete, namely, creating hormones, muscle, and other proteins.
Can Maltose Be Considered An Enzyme?
Before we dive into the meat of this question, let’s first discuss what an enzyme is.
What Are Enzymes?
Remember when I told you what metabolism is? That it’s a collection of chemical reactions on a cellular level? Well, enzymes are proteins that help to speed up these chemical processes.
They’re essential to tons of bodily functions including digestion, liver health, breathing, and nerve function, but much like anything in this life, enzymes are all about balance. A surplus or dearth of enzymes can be severely detrimental to our health.
Our bodies naturally produce enzymes, but they can also be manufactured using genetically modified organisms.
The eagle eyes among you will have noticed that I dropped the P-word (protein), and will have used the logic from the carb vs protein debate to deduce maltose isn’t an enzyme as a carb cannot be a protein. But why then do people confuse the two beasts?
Untangling Maltose and Enzymes
The reason maltose is sometimes mistaken for an enzyme is that enzymes play a big role in the creation of maltose.
Earlier I mentioned something called amylase and how it’s responsible for most of the maltose formation in the mashing stage of the brewing process. Amylase is an enzyme. It catalyzes the hydrolysis of starch, which basically means it cleaves it, breaking it down into sugars, i.e. the carb of the hour…maltose.
So, no, maltose isn’t an enzyme, but enzymes are essential to its creation.
Does Yeast Completely Digest Maltose?
People’s first reaction to this mysterious byproduct of the brewing process is to feel a little violated, and I get it; you want to know what’s in your drink before you drink it, but here’s the thing…there is little to no maltose in alcohol.
The yeast gobbles it all up in the fermenting process, leaving mostly just ethanol and carbon dioxide.
There is undoubtedly trace amounts of the carb left in our favorite drinks, but realistically, it’s not going to fiddle with our blood sugar or contribute to a beer belly.
Will Maltose Dissolve in Water?
Maltose does indeed dissolve in water because it’s composed of polar molecules, and water is a polar solvent. The water forms hydrogen bonds with the maltose molecules, essentially blurring the lines between the substances.
What Are Polar And Nonpolar Substances?
Just like the Earth, certain molecules have poles, one with a positive charge and one with a negative charge. Polar molecules are attracted to other polar molecules, allowing the two to blend seamlessly into one another.
Although nonpolar molecules are fairly attracted to one another, they cannot mix with polar molecules. A famous example of this mismatch would be water and oil. Oil is made up of nonpolar molecules, which is why it separates from water.
Summing Up With 15 Awesome Facts About Maltose
- Maltose is composed of two glucose units fused by a glycosidic linkage, meaning it’s a disaccharide.
- It’s roughly one-third as sweet as regular table sugar.
- Maltose is predominantly found in grains.
- Maltose molecules have a pyranose form.
- It’s used to brew beer but is mostly absorbed by the yeast during fermentation.
- Maltose is produced when the enzyme amylase cleaves starch.
- It’s a carbohydrate.
- As far as we know, it’s not bad for us in moderation.
- As it’s made up of polar molecules, it dissolves in water, a polar solvent.
- It helps to give bread an enticing brown hue.
- Maltose is a reducing sugar, which means that the molecules can form chemical reactions with surrounding molecules.
- As it has zero fructose units, it’s theoretically better for our health than table sugar.
- It occurs naturally in certain fruits and vegetables.
- It’s found in yams, but only after they’re cooked.
- Maltose is utilized as an inexpensive alternative to standard table sugar.
